Services
Our regulatory frameworks are built to align with your current phase and expand as your operations scale.
Our regulatory frameworks are built to align with your current phase and expand as your operations scale.
Our regulatory consultancy service is built on the ARC Methodology — a structured, risk-based approach designed to uncover gaps, reduce exposure, and build robust compliance systems that scale with your business.
We start with a detailed ARC scorecard assessment, followed by a hands-on gap analysis and risk review. From there, we deliver a practical, prioritised report with clear next steps tailored to your product category and market ambitions.
We support a wide range of regulated industries and provide technical services including:

Built for founders and brands in regulated markets who want to move fast — without cutting corners.
A practical framework that helps challenger brands scale safely and stay ahead of enforcement risks.
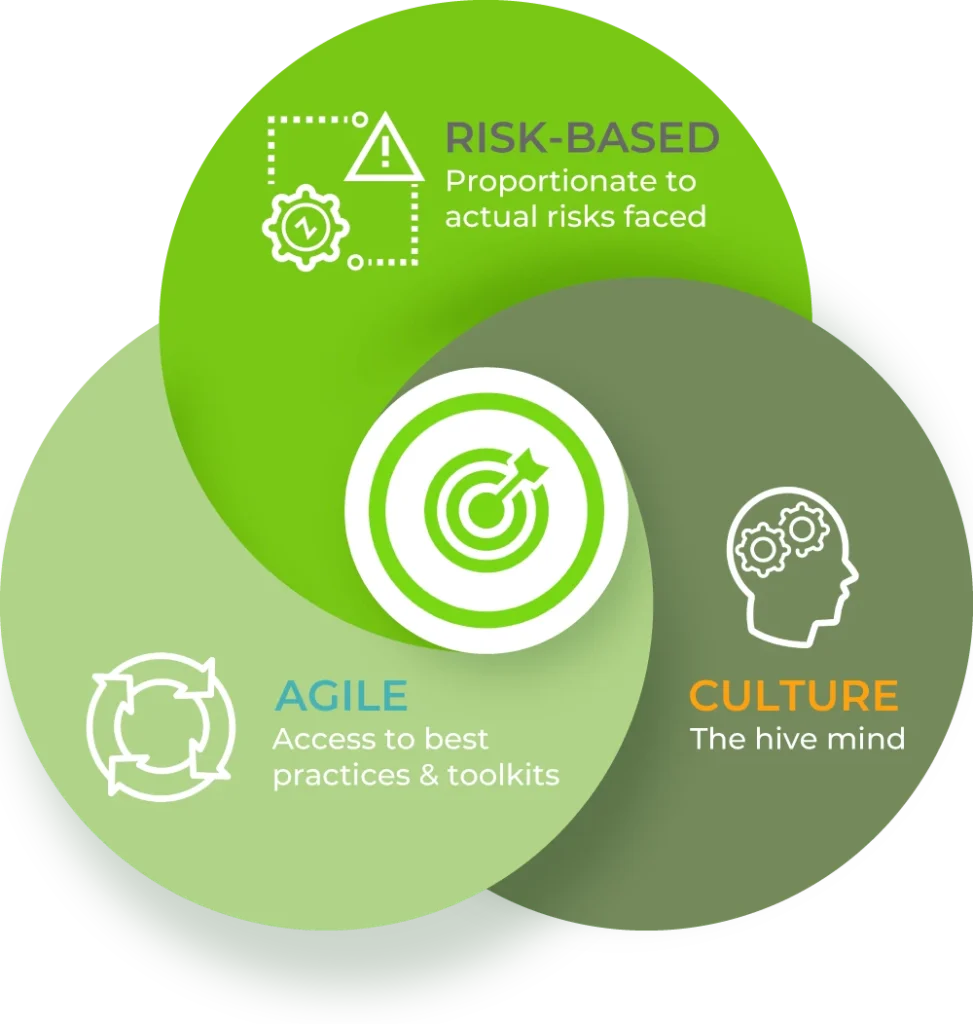
Agile Systems — Lean, scalable, zero bloat.
Risk-Based — Focus only on what actually matters.
Compliance Culture — Make it part of how your team works, not just paperwork.
Get it wrong: fines, recalls, bans, brand damage. Get it right: protect growth, unlock markets, impress partners.
👉 Take the free ARC Scorecard or book a discovery call.